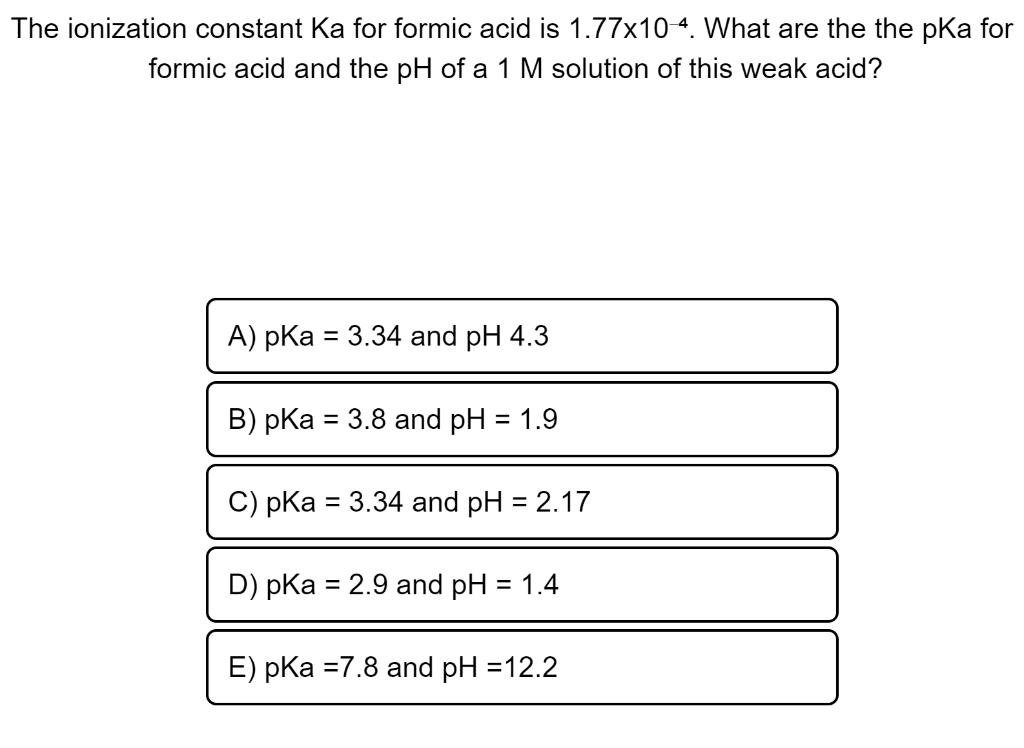
Energies | Free Full-Text | Cost Efficiency Analysis of H2 Production from Formic Acid by Molecular Catalysts

Dissociation constant (K_a) of formic acid and acetic acid are 2.5xx10^-4 and 0.5xx10^-5 respect... - YouTube

The Ka values of formic acid and acetic acid are respectively 1.77 × 10^-4 and 1.75 × 10^-5 . The ratio of the acid strength of 0.1M acid is:

What is the pH of a 0.15 M solution of formic acid, HCOOH ? `{:("Formic Acid ",K_a),(HCOOH - YouTube

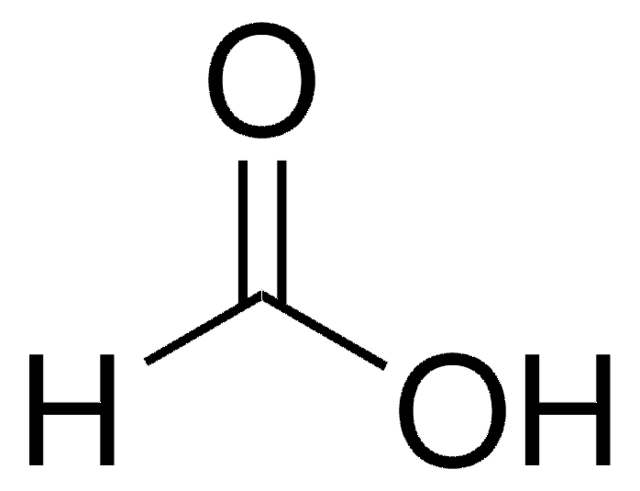
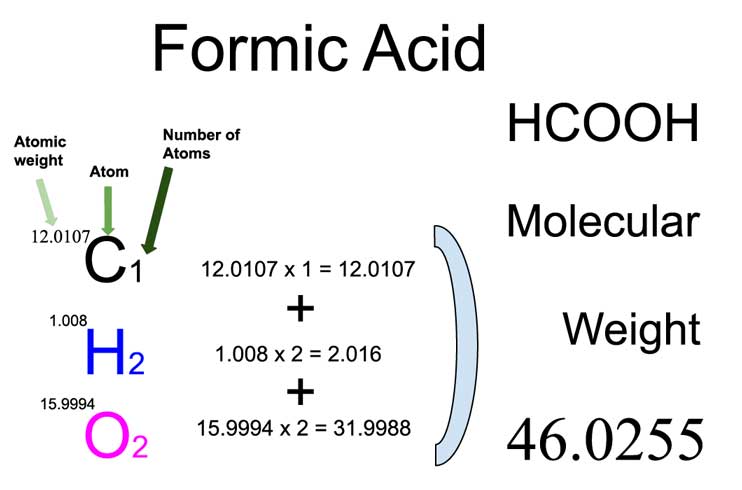
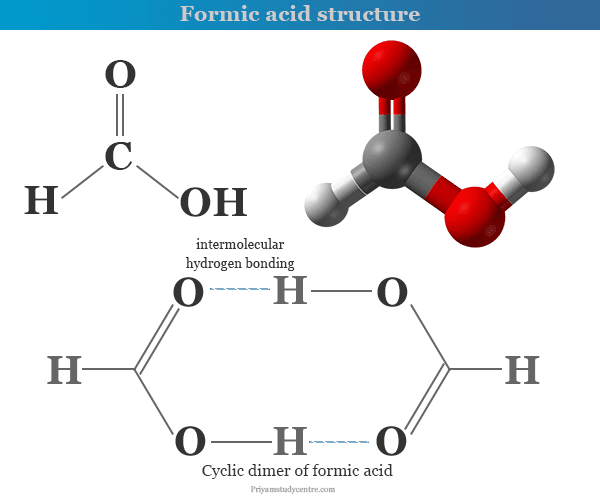
Formic Acid (HCOOH) - Structure, Molecular Mass, physical Properties, chemical properties, Uses and FAQs of formic acid (HCOOH)
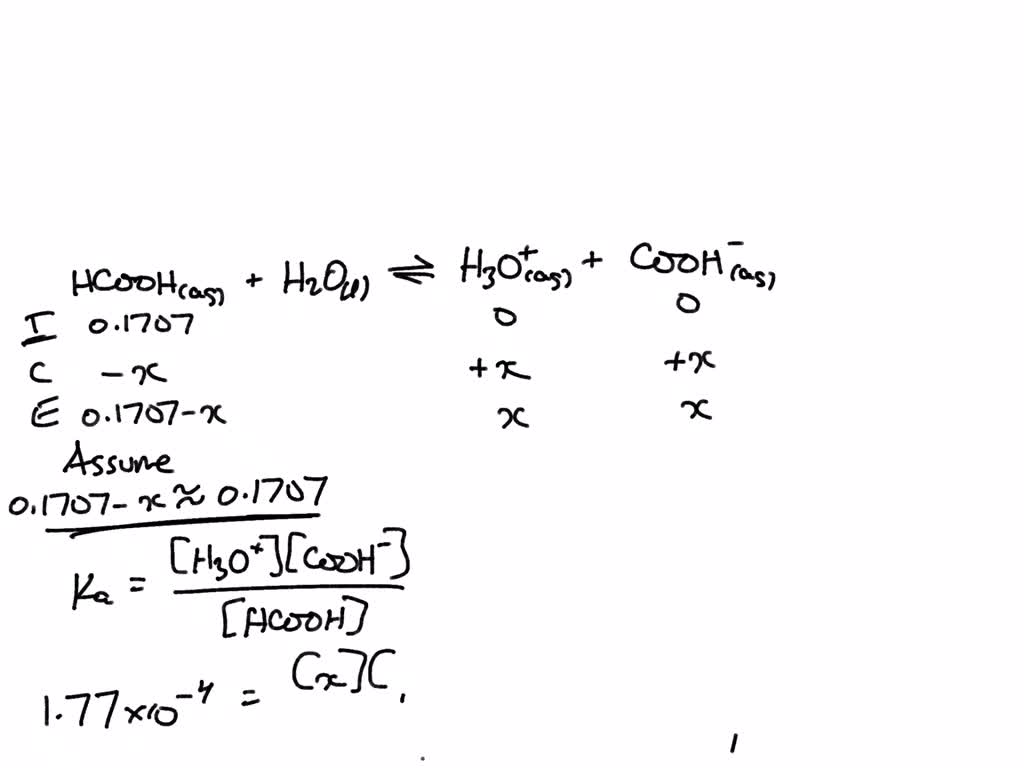
SOLVED: Formic acid is a weak acid with the formula HCOOH; the value of Ka for formic acid is 1.77 x 10-4 In aqueous solution, formic acid partially dissociates according to the

When a solution of formic acid was titrated with KOH solution, the pH of the solution was 3.65 when half the acid was neutralized. Calculate Ka(HCOOH) .
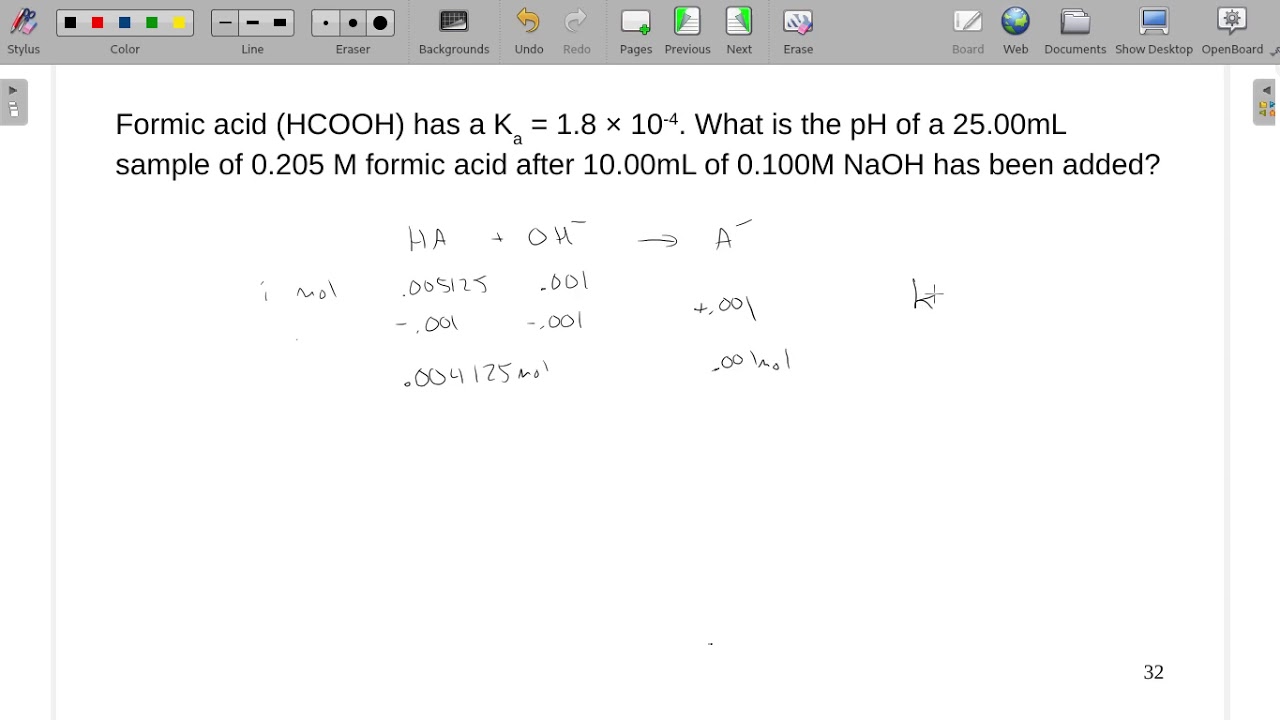
Formic acid has a Ka = 1.8×10-4. What is the pH of a 25.00mL sample of 0.205 M formic acid after... - YouTube

Dehydrogenation of Formic Acid by a RuII Half Sandwich Catalyst - Vatsa - 2021 - ChemistrySelect - Wiley Online Library